NIDEK announces FDA 510(k) clearancefor the AFC-330 – Non-Mydriatic Auto Fundus Camera
2012.05.18
– May 18, 2012, Gamagori, JAPAN –
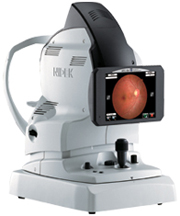
NIDEK, a global leader in the design, manufacturing, and distribution of ophthalmic equipment, announces the FDA 510(k) clearance in the US for the AFC-330, their most automated fundus camera yet.
“The AFC-330 represents NIDEK’s 3rd generation of automated fundus camera. We are both proud and excited to be leading the way designing and producing fundus cameras that are faster, easier, and more versatile than ever. We anticipate increasing our fundus camera market share with our market expansion” Motoki Ozawa, President and CEO of NIDEK.
The AFC-330 makes quantum leaps improving the operator and patient interface, simplicity, automation, and total practice efficiencies. This camera offers an all in one compact design, auto alignment on the X-Y-Z axis, and a wide range of automated features including auto stereo for Glaucoma Management. The lower flash intensity and sound-dampening internal movements mean less retakes and improved patient comfort. No other Non-Mydriatic camera provides both this level of advanced automation and image quality.
NIDEK will continue to provide products, which meet the diverse needs of eye care professionals all over the world.
For further information contact:
Public Relations, Planning Dept.
NIDEK CO., LTD.
Email: info@nidek.co.jp
NOTE: The availability of the products differs from country to country depending on the status of regulatory approval in each country. Specifications and design are subject to change.
Back to List





















 TOP
TOP