NIDEK launches the GS-1 Gonioscope
2018.09.20
-September 20, 2018, Gamagori, JAPAN-
NIDEK CO., LTD., a global leader in the design, manufacturing, and distribution of ophthalmic, optometric, and lens edging equipment, is pleased to announce the launch of the GS-1 Gonioscope.
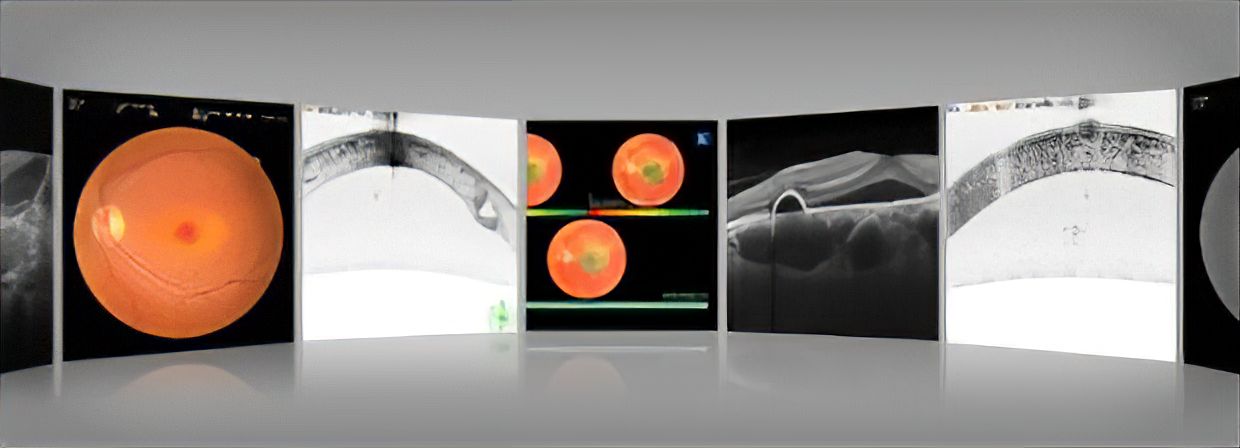
This groundbreaking device represents a first in ophthalmology from a company known for innovation. The GS-1 is the first automated gonioscopy device enabling 360-degree color iridocorneal angle imaging allowing more clinicians to reliably perform gonioscopy in daily practice. By increasing the number of clinicians who will perform gonioscopy with the GS-1, pathology may be detected in a timely fashion and postoperative follow up could be performed by referring physicians.
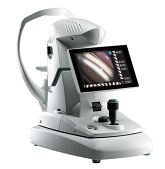
The GS-1 was developed to capture the entire 360 degrees of the angle using a unique 16 surface multi-mirrored prism lens. Optimization of this lens combined with projection of a white LED into the angle simulates indirect static gonioscopy.
Automated Angle Detection provides guidance for capturing the iridocorneal angle. For patient comfort, this system uses gel coupling. Once correctly aligned, the autoshot initiates imaging of the angle structures. Each area is automatically captured in 17 different foci, enabling versatile approaches to the angle.
The acquired images can be automatically stitched together, and linear and circular montages of the entire 360 degrees are presented for examination. This innovative “Stitching” function provides a view of the entire angle to support angle assessment and clinical findings.
Different from classic gonioscopy, patient diagnosis can occur after image acquisition rather than at the time of assessment. Images can be easily shared with referring physicians and used for patient education.
“We believe that the GS-1 represents a new chapter in the history of ophthalmology. Especially in light of recent advances such as MIGS, the GS-1 is exactly the innovation that will support surgical assessment in an objective and efficient manner. The GS-1 also shows NIDEK’s commitment to continually innovate to present physicians with devices that will enhance clinical practice.” says Motoki Ozawa, President and CEO of NIDEK CO., LTD.
Product/Model name: GONIOSCOPE GS-1
https://www.nidek-intl.com/product/ophthaloptom/diagnostic/dia_retina/gs-1.html
For further information contact:
International Planning Dept.,
NIDEK CO., LTD.
Email: info@nidek.co.jp
NOTE: The availability of the products differs from country to country depending on the status of regulatory approval in each country. Specifications and design are subject to change without notice.
Back to List





















 TOP
TOP