NIDEK’s MP-3 Microperimeter received 510K clearance
2016.11.17
-November 7, 2016, Gamagori, Japan-
NIDEK CO., LTD., a global leader in the design, manufacture, and distribution of ophthalmic, optometric, and lens edging equipment, is pleased to announce that the United States Food & Drug Administration (FDA) has issued 510K Clearance for the MP-3 Microperimeter.
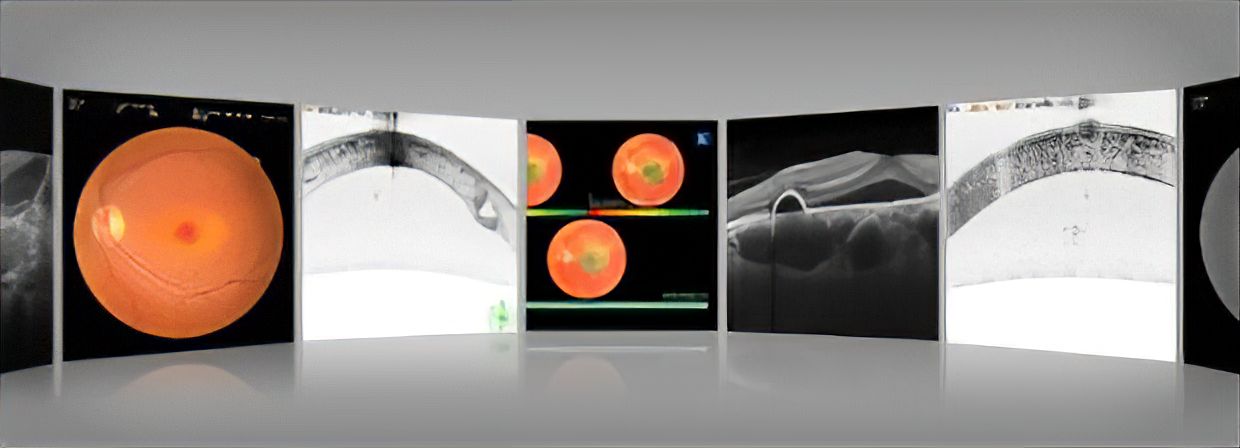
Assessment of retinal morphology has advanced significantly due to the introduction of Optical Coherence Tomography into clinical practice. Advances in the functional evaluation of retinal pathology are due to the use of the microperimeter. The MP-3 measures local retinal sensitivity for functional assessment of the retina. The results can be displayed over a color fundus image, correlating retinal anatomy to retinal function.
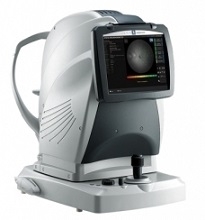
For enhanced clinical assessment, the MP-3 now includes a wider range of stimulus intensity, from 0 to 34 dB, compared to the MP-1. The MP-3 measures perimetric threshold values, even for normal eyes. A maximum stimulus luminance of 10,000 asb allows evaluation of low-sensitivity.
The NIDEK auto tracking and auto alignment functions provide more accurate measurements increasing patient and operator comfort and efficiency. These functions allow easy follow-up and reduce variations between examiners, resulting in well-aligned follow up exams.
Retinal morphology can be evaluated with the 12-megapixel fundus camera included the MP-3 unit which acquires high resolution images of retinal pathology and allows easy image acquisition.
Product / Model name: Microperimeter MP-3
For further information contact:
Public Relations, Planning Dept.
NIDEK CO., LTD.
Email: info@nidek.co.jp
Back to List





















 TOP
TOP