NIDEK’s RS-3000 Advance Optical Coherence Tomographer received 510K clearance
2014.03.07
– March 7, 2014, Gamagori, JAPAN –
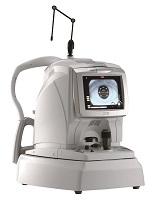
NIDEK, a global leader in the design, manufacturing, and distribution of ophthalmic equipment, is pleased to announce FDA clearance of the RS-3000 Advance. This premier OCT system incorporating Scanning Laser Ophthalmoscope is designed for comprehensive evaluation of the retina and choroid. The RS-3000 Advance provides exquisite detail of the retinal and choroidal microstructures to assist in clinical diagnosis.
Features include the choroidal mode, which allows detailed evaluation of the choroid. A wide area scan ensures excellent overall coverage of the retinal structures. Microsaccades and other involuntary eye movements are compensated by the Tracing HD function during the macular line scans. This function ensures accurate alignment of up to 120 macular line scan images for enhanced image averaging. The Torsion Eye Tracer (TET) function compensates for cyclotorsion during image acquisition enhancing the quality of follow-up data.
High speed scanning of 53,000 Ascans/sec during acquisition with tracing increases accuracy as well as two additional higher sensitivity scanning modes with tracing enables imaging through media opacities.
Mr. Motoki Ozawa, President of NIDEK stated, “We are pleased to have this clearance, and confident the results reflect the quality of our diagnostic tools in imaging that assist doctors in diagnosing ocular diseases”.
Back to List





















 TOP
TOP